Answer:
Q = 62383.44 Joules
Step-by-step explanation:
Given that,
Mass of water, m = 710 gm
Initial temperature of water,
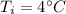
Final temperature of water,
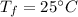
The specific heat capacity of liquid water is,
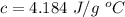
Heat transferred is given by :

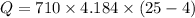
Q = 62383.44 Joules
So, the amount of heat transferred is 62383.44 Joules. Hence, this is the required solution.