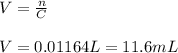Answer:
11.6 mL
Step-by-step explanation:
First we need to calculate the number of moles of Zn2+ present in the solution:
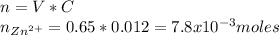
As the charge of ion zinc is 2+ and the charge of hydroxide is 1-, we need double moles of NaOH:
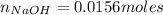
As we have the concentration and the moles, we can calculate the volume: