Answer:
49.3% 81Br and 50.7% 79Br or
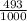 81Br and
81Br and
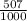 79BR
79BR
Explanation:
To get the fraction-of-occurrences of the isotopes we must write the following equation. x is the isotopic abundance of 81Br, we can use 1 - x to get the isotopic abundance of 79Br.
(78.918)(1 - x) + (80.916)(x) = 79.903
78.918 - 78.918x + 80.916x = 79.903
1.998x = 0.985
x = 0.493
0.493 × 100 = 49.3% 81Br
100 - 49.3 = 50.7% 79Br
49.3% 81Br and 50.7% 79Br or
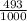 81Br and
81Br and
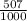 79BR
79BR