Answer:
Option D, There will be an increase of
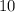 degree Celsius in the temperature of the water
degree Celsius in the temperature of the water
Step-by-step explanation:
As we know -

Where Q is the total amount of heat produced
m signifies mass of any substance
C signifies specific heat
and dT represents change in temperature
Specific heat of water is 1 calories per gram per degree Celsius
On substituting the given values in above equation, we get -
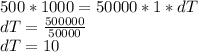
Hence , there will be an increase of approximately
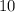 degree Celsius in the temperature of the water
degree Celsius in the temperature of the water