Answer:
The volume of a chlorine solution a homeowner should add to her swimming pool is
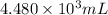 .
.
Step-by-step explanation:
The solution contains 5.50 percent chlorine by mass.
Mass of the chlorine required to added in the pool= x
Mass of the water in the pool = y
Volume of the water ,V=
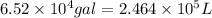
(1 gal = 3.78 L)
V =
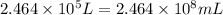
( 1 L = 1000 mL)
Density of the water ,d= 1.00 g/mL
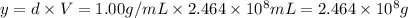
y =
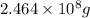
1.00 g of chlorine per million grams of water .
Then for
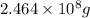 of water, we required x amount of chlorine:
of water, we required x amount of chlorine:
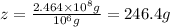
x = 246.4 grams of chlorine
Mass of the chlorine solution = M'
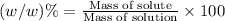
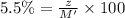
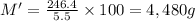
Density of the chlorine solution = D = 1 g/mL
Volume of the chlorine solution to be added in the pool : v
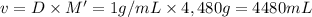
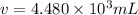
The volume of a chlorine solution a homeowner should add to her swimming pool is
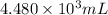 .
.