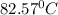Answer: The boiling point of an benzene solution is
Step-by-step explanation:
Depression in freezing point is given by:
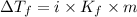
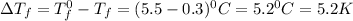 = Depression in freezing point
= Depression in freezing point
i= vant hoff factor = 1 (for non electrolyte)
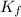 = freezing point constant =
= freezing point constant =
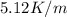
m= molality
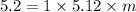
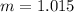
Elevation in boiling point is given by:
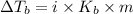
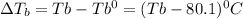 = elevation in boiling point
= elevation in boiling point
i= vant hoff factor = 1 (for non electrolyte)
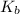 = boiling point constant =
= boiling point constant =
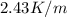
m= molality = 1.015
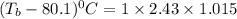
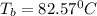
Thus the boiling point of an benzene solution is