Step-by-step explanation:
The given data is as follows.
Volume of lake =
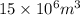 =
=
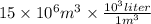
Concentration of lake = 5.6 mg/l
Total amount of pollutant present in lake =
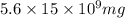
=
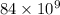 mg
mg
=
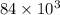 kg
kg
Flow rate of river is 50
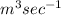
Volume of water in 1 day =
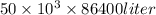
=
 liter
liter
Concentration of river is calculated as 5.6 mg/l. Total amount of pollutants present in the lake are
 or
or
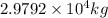
Flow rate of sewage =
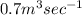
Volume of sewage water in 1 day =
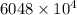 liter
liter
Concentration of sewage = 300 mg/L
Total amount of pollutants =
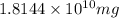 or
or
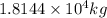
Therefore, total concentration of lake after 1 day =
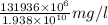
= 6.8078 mg/l
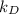 = 0.2 per day
= 0.2 per day
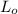 = 6.8078
= 6.8078
Hence,
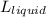 =
=
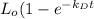
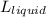 =
=
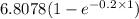
= 1.234 mg/l
Hence, the remaining concentration = (6.8078 - 1.234) mg/l
= 5.6 mg/l
Thus, we can conclude that concentration leaving the lake one day after the pollutant is added is 5.6 mg/l.