Answer:
Choice A:
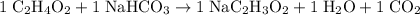 .
.
Step-by-step explanation:
Indeed it is possible to balance this equation by the conservation of atoms in a chemical reaction. However, knowing what's actually going on in this process will likely make this problem easier to solve.
Vinegar contains acetic acid
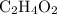 . Acetic acid is a monoprotic acid. In other words, each
. Acetic acid is a monoprotic acid. In other words, each
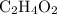 can dissociate to produce up to one hydrogen ion
can dissociate to produce up to one hydrogen ion
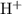 . That is:
. That is:
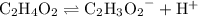 .
.
Baking soda is a common name for sodium bicarbonate
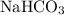 . Each formula unit of
. Each formula unit of
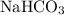 contains one bicarbonate ion:
contains one bicarbonate ion:
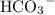 . Each bicarbonate ion will consume one hydrogen ion to produce water and carbon dioxide:
. Each bicarbonate ion will consume one hydrogen ion to produce water and carbon dioxide:
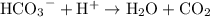 .
.
For this chemical equation to balance, the number of hydrogen ions that
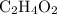 supplies shall be the same as the number of these ions that
supplies shall be the same as the number of these ions that
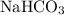 consumes. Each unit of
consumes. Each unit of
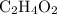 supplies one unit of hydrogen ions while each unit of
supplies one unit of hydrogen ions while each unit of
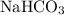 consumes one unit of hydrogen ions. Reacting the two at a one-to-one ratio will make sure that this reaction neither run short of hydrogen ions or produce more hydrogen ions than it need.
consumes one unit of hydrogen ions. Reacting the two at a one-to-one ratio will make sure that this reaction neither run short of hydrogen ions or produce more hydrogen ions than it need.
Hence the coefficient in front of
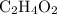 and
and
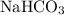 shall be the same. Let their coefficients be one.
shall be the same. Let their coefficients be one.
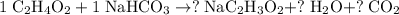 .
.
Now, balance this equation with reference to the number of atoms:
- One Na atom;
- Five H atoms;
- Five O atoms;
- Three C atoms.
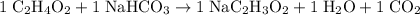 .
.