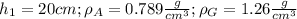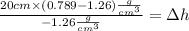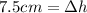Answer:
7.5 cm
Step-by-step explanation:
In the figure we can see a sketch of the problem. We know that at the bottom of the U-shaped tube the pressure is equal in both branches. Defining
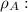 Ethyl alcohol density and
Ethyl alcohol density and
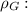 Glycerin density , we can write:
Glycerin density , we can write:
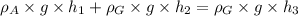
Simplifying:
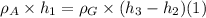
On the other hand:
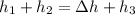
Rearranging:
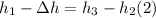
Replacing (2) in (1):
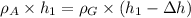
Rearranging:
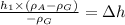
Data: