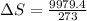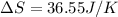Answer:
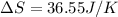
Step-by-step explanation:
As we know that entropy change for phase conversion is given as
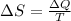
Here we know that heat required to change the phase of the ice is given as
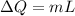
here we have
m = 29.8 g = 0.0298 kg
L = 335000 J/kg
now we have
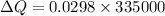
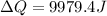
also we know that temperature is approximately same as freezing temperature
so we have
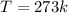
so here we have