Step-by-step explanation:
Since, it is known that number of moles equal to the mass divided by the molar mass of a substance.
As molar mass of NaCl is 58.44 g/mol. Hence, number of moles of NaCl present in 158.84 g are calculated as follows.
No. of moles =

=
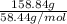
= 2.715 mol
Therefore, there are 2.715 moles present in 100 ml solvent.
Hence, the molar concentration will be as follows.
Molar concentration =
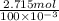 mol/liter
mol/liter
= 27.15 M
It is given that error in weight is 0.005 g and error in volume is 0.2 ml.
Hence, total volume will be (100ml + 0.2 ml) = 100.2 ml.
Therefore, now molar concentration will be as follows.
Molar concentration =
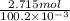 mol/liter
mol/liter
= 27.09 M
Thus, calculate the deviation as follows.
(27.15 - 27.09) mol/liter
= 0.06 mol/liter
Hence, we can conclude that the molar concentration of sodium chloride 27.15 M and its associated standard deviation is 0.06 mol/liter.