Answer:
Order of increasing strength of intermolecular attraction:
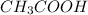 >
>
 >
>
 > Ar
> Ar
Step-by-step explanation:
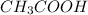 can form hydrogen bond as H atom is attached with electronegative atom O.
can form hydrogen bond as H atom is attached with electronegative atom O.
Rest three,
 ,
,
 , Ar are non-polar molecules.
, Ar are non-polar molecules.
In non-polar molecules, van der Waal's intermolecular forces of attractions exist. Hydrogen bonding is stronger intermolecular attraction then van der Waal's intermolecular forces of attraction, hence,
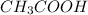 has strongest intermolecular attractions.
has strongest intermolecular attractions.
Ar will have least intermolecular attraction, as it behaves almost as ideal gas and there is no intermolecular attraction exist between molecules of ideal gases.
Molecular size and mass of
 is high as compared to
is high as compared to
 .
.
van der Waals intermolecular forces of attraction increases with increase in size.
Therefore,
Order of increasing strength of intermolecular attraction will be:
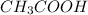 >
>
 >
>
 > Ar
> Ar