Answer:
ρm=4.14g/ml :metal density
Step-by-step explanation:
Conceptual analysis
We apply the formula to calculate the density:
ρ= m /V Formula (1)
Where:
ρ:density in g/mL
m: mass in g (grams)
V= volume in ml (milliliters)
Known data
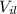 : final volume of liquid=55.25 ml
: final volume of liquid=55.25 ml
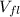 : final volume of liquid=61.00 ml
: final volume of liquid=61.00 ml
ms: metal mass=23.795 g
Problem development
The volume of the metal (vm) is equal to the change in volume of the liquid.
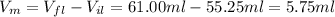
We replace the data in formula (1 )to calculate the density of the metal(ρm):
ρm= mm /Vm=23.795 g/5.75ml
ρm=4.14g/ml