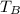Answer:
Step-by-step explanation:
Gas law equation for any gas is as follows .
PV = nRT ( for n moles of gas )
Let the pressure , volume , temperature and mole of gas in cylinder A be P,V
T₁ and 2n . Pressure , volume , temperature and mole in cylinder B will be
P , V , T₂ and n.
Applying Gas laws , we get
For gas in cylinder A
PV = 2n R T₁
For gas in cylinder B
PV = n R T₂
Equating these equations , we get
2n R T₁ = n R T₂
2 T₁ = T₂
or,
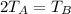
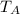 is less than
is less than