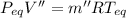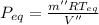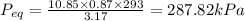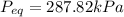Answer:
Volume of Tank 2, V' =
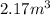
Equilibrium Pressure,
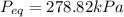
Given:
Volume of Tank 1, V = 1

Temperature of Tank 1, T =
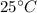 = 298 K
= 298 K
Pressure of Tank 1, P = 500 kPa
Mass of air in Tank 2, m = 5 kg
Temperature of tank 2, T' =
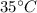 = 303 K
= 303 K
Pressure of Tank 2, P' = 200 kPa
Equilibrium temperature,
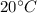 = 293 K
= 293 K
Solution:
For Tank 1, mass of air in tank can be calculated by:
PV = m'RT
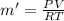
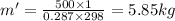
Also, from the eqn:
PV' = mRT
V' = volume of Tank 2
Thus
V' =
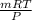
V' =
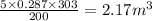
Now,
Total Volume, V'' = V + V' = 1 + 2.17 = 3.17

Total air mass, m'' = m + m' = 5 + 5.85 = 10.85 kg
Final equilibrium pressure, P'' is given by: