Answer:
310.69K
Step-by-step explanation:
Given parameters:
Initial temperature T₁ = 292K
Initial pressure P₁ = 1.25atm
Final pressure P₂ = 1.33atm
Unknown:
Final temperature T₂ = ?
Solution
To find the unkown, we need to apply the combined gas law. From the combined gas law, it can be deduced that at constant volume, the pressure of a give mass or mole of gas varies directly with the absolute temperature.
Since the same aerosol can is heated, the volume is constant.
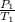 =
=
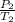
Now, we have to make T₂ the subject of the formula:
T₂ =
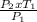
T₂ =
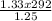 = 310.69K
= 310.69K