Answer: (E) 300 bq
Step-by-step explanation:
Half life is the amount of time taken by a radioactive material to decay to half of its original value.
Radioactive decay process is a type of process in which a less stable nuclei decomposes to a stable nuclei by releasing some radiations or particles like alpha, beta particles or gamma-radiations. The radioactive decay follows first order kinetics.
Half life is represented by
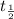
Half life of Thallium-208 = 3.053 min
Thus after 9 minutes , three half lives will be passed, after ist half life, the activity would be reduced to half of original i.e.
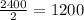 , after second half life, the activity would be reduced to half of 1200 i.e.
, after second half life, the activity would be reduced to half of 1200 i.e.
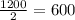 , and after third half life, the activity would be reduced to half of 600 i.e.
, and after third half life, the activity would be reduced to half of 600 i.e.
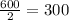 ,
,
Thus the activity 9 minutes later is 300 bq.