Answer:
The two changing properties in Charles’ law are temperature and volume.
Step-by-step explanation:
Charles’ law state the presence of direct relationship between temperature and volume of the system of gas molecules at constant pressure condition. In this law, the expansion of gas has been explained with the increase of temperature.
As the temperature is increased or the system of gas molecules are heated, the gas molecules tend to expand their volume to maintain the pressure same.
So the temperature and volume are directly proportional at constant pressure. Thus the two changing properties of Charles’ law is temperature and volume. The mathematical representation of Charles’ law is
V∝T (at constant Pressure)
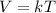
Here k is the non-zero constant and V and T are volume and temperature respectively.