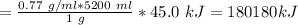Answer:
Math expression:
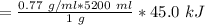
Step-by-step explanation:
Given:
Energy produced per gram of gasoline = 45.0 kJ
Density of gasoline = 0.77 g/ml
Volume of gasoline = 5.2 L=5200 ml
To determine:
The amount of energy produced by burning 5.2 L gasoline
Calculation set-up:
1. Calculate the mass (m) of gasoline given the density (d) and volume (v)
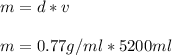
2. Calculate the amount of energy produced