Answer : The molar mass of unknown compound is 128.22 g/mole
Explanation :
Mass of unknown compound = 9.72 g
Mass of solvent = 50.0 g
Formula used :
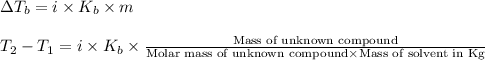
where,
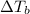 = elevation in boiling point
= elevation in boiling point
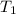 = temperature of solvent =
= temperature of solvent =
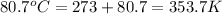
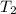 = temperature of solution =
= temperature of solution =
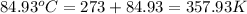
i = Van't Hoff factor = 1 (for non-electrolyte)
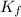 = boiling point constant for solvent = 2.79 K/m
= boiling point constant for solvent = 2.79 K/m
m = molality
Now put all the given values in this formula, we get:
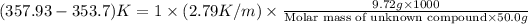
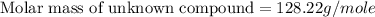
Therefore, the molar mass of unknown compound is 128.22 g/mole