Answer: Option (c) is the correct answer.
Step-by-step explanation:
A compound is defined as the substance in which different elements are chemically combined together in a fixed ratio by mass.
For example,
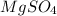 is a compound and elements are present in 1:4 ratio.
is a compound and elements are present in 1:4 ratio.
A compound can be divided into its constituent or simpler substances.
Hence, a chemical formula shows the elements involved in it along with their identity and relative proportion.
But a chemical formula does not tell how atoms are joined together as it can be done by structural formula and not by chemical formula.
Thus, we can conclude that the chemical formula of a compound does not indicate how elements are joined in the compound.