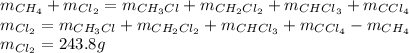Answer:
How many grams of CCL4 were formed? 116.9 g
How many grams of Cl2 reacted with the CH4? 243.8 g
Step-by-step explanation:
First we need to know the molar mass for every element or compound in the reaction:

Now we proceed to calculate the amount of moles produced, per product:
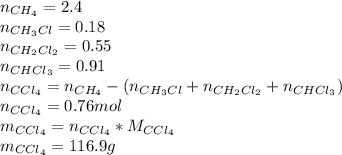
To calculate the mass of chlorine we just need to make a mass balance: