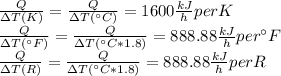Answer:
1600 kJ/h per K, 888.88 kJ/h per °F and 888.88kJ/h per R
Step-by-step explanation:
We make use of relations between temperature scales with respect to degrees celsius:
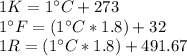
This means that a change in one degree celsius is equivalent to a change of one kelvin, while for a degree farenheit and rankine this is equivalent to a change of 1.8 on both scales.
So: