Answer:
Step-by-step explanation:
(a)
Gold has an atomic mass of 197 g/mol means 197 g of gold contains 6.023×10^{-23} atoms.
therefore number of atoms in 17.4 g is
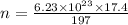
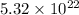
Number of protons in each atom is 79.
therefore Total number of protons is 5.32×10^{22}×79 =4.2×10^24. protons
Their total positive charge is 4.2×10^{24}×1.6×10^{-19) =6.72×10^5 C.
(b)
equal number of protons should be there to neutralize the material therefore number of electrons is 4.2×10^(24.)