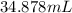Answer:
Volume of
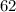 % acid solution is
% acid solution is
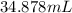
Step-by-step explanation:
The total weight of
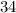 % mixture containing
% mixture containing
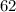 % and
% and
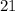 % solutions
% solutions
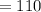 mL
mL
Let the weight of
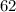 % solution is equal to
% solution is equal to
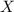 mL
mL
then the weight of
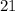 % solution is equal to
% solution is equal to
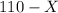 mL
mL
Arranging the given conditions in the form of equation, we get -
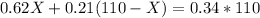
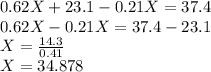 mL
mL
The volume of
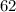 % acid solution is
% acid solution is