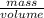Answer:
Step-by-step explanation:
I could have set up an experiment to determine the density of the metal in the jar. When I derive the density of the metal, I will compare it with that of every other metal to see if it properly fits any.
Density is the mass per unit volume of substance. It is the amount of substace contained per unit volume.
To find the density of the metal, the mass of the metal is obtained by directly weighing it. The volume is found by immersing the metal in water as it will sink. The volume of the water displaced is the volume of the metal. A measuring cylinder or an overflow is used to determine the volume of the liquid displaced.
Then:
Density =