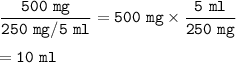The volume of the suspension required : 10 ml
Further explanation
Given
0.500 g of ampicillin
The liquid suspension concentration = 250 mg/5 mL
Required
The volume of the suspension required
Solution
The concentration of a substance can be expressed in various units such as molarity, ppm, g / L etc.
Each unit change, we must pay attention to the conversion factor
Conversion g to mg
0.5 g = 500 mg
The volume of the suspension needed :
=mass x concentration