Mass of Barium chloride produced : 2666.56 g
Further explanation
Given
Reaction
6 NaCl + Ba3(PO4)2 → 2 Na3PO4 + 3 BaCl2
1500 g of sodium chloride
Required
Barium chloride produced
Solution
mol NaCl (MW= 58.5 g/mol) :
mol = mass : MW
mol = 1500 g : 58.5 g/mol
mol = 25.64
mol Barium chloride-BaCl2 based on mol NaCl(as a limiting reactant)
From the equation, mol ratio NaCl : BaCl2 = 6 : 3, so mol BaCl2 :
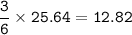
mass BaCl2(MW=208 g/mol) :
mass = mol x MW
mass = 12.82 x 208
mass = 2666.56 g