Answer: There is no solution for the required amount of butanoic acid.
Step-by-step explanation:
We are given:
Mass of butanoic acid needed = 60.00 grams
36.9 % w/w butanoic acid solution
This means that 36.9 grams of butanoic acid is present in 100 grams of solution
Applying unitary method:
If 36.9 grams of butanoic acid is present in 100 grams of solution
So, 60.00 grams of butanoic acid will be present in =
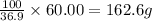
As, the given amount of solution is less than the required amount.
Hence, there is no solution for the required amount of butanoic acid.