Answer:
initially it will form precipitate and with excess of ammonia it will form soluble product.
Step-by-step explanation:
The reaction of nickel with ammonia is:
With aqueous ammonia nickel precipitate out as green gelatinous Ni(OH)₂:
[light green]
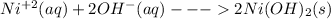 (green colored).
(green colored).
If we add further more of ammonia, it will dissolve to form blue solution as:
![Ni(OH)_(2)(s) + 6NH_(3)(aq) <==> [Ni(NH_(3))_(6)]^(+2)(aq) + 2OH^(-)(aq)](https://img.qammunity.org/2020/formulas/chemistry/middle-school/yw4w9y1jwvq6nh2ldr4cq6lie5thev0kxm.png)
So initially it will form precipitate and with excess of ammonia it will form soluble product.