Answer:
78.4g
Step-by-step explanation:
Given parameters:
Mass of copper (II) chloride = 90g
Mass of sodium nitrate = 120g
Unknown:
Mass of sodium chloride that can be formed = ?
Solution:
The balanced chemical reaction is:
CuCl₂ + 2NaNO₃ → Cu(NO₃)₂ + 2NaCl
Let us find the limiting reactant. This reactant will determine the extent of the reaction of the amount of product that will be formed.
First, convert the masses to number of moles;
Number of moles =
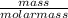
Molar mass of CuCl₂ = 63.5 + 2(35.5) = 134.5g/mol
Molar mass of NaNO₃ = 23 + 14 + 3(16) = 85g/mol
Number of moles of CuCl₂ =
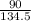 = 0.67mole
= 0.67mole
Number of moles of NaNO₃ =
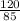 = 1.41mole
= 1.41mole
From the balanced reaction equation:
1 mole of CuCl₂ would react with 2 moles of NaNO₃
0.67mole of CuCl₂ would require 0.67 x 2 = 1.34mole of NaNO₃
So, CuCl₂ is the limiting reactant
1 mole of CuCl₂ will produce 2 mole of NaCl
0.67mole of CuCl₂ will therefore yield 0.67 x 2 = 1.34mole of NaCl
Mass of NaCl = number of moles x molar mass
Molar mass of NaCl = 23 + 35.5 = 58.5g/mol
Mass of NaCl = 58.5 x 1.34 = 78.4g