Carbon burns in the presence of oxygen to give carbon dioxide. The chemical equation describing this reaction is
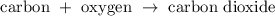
Answer: Option B
Explanation:
The chemical equations are the representation of a particular reaction.This equation used to avoid the description of the reaction and narrowing it to precise statement. It consists of two parts namely,
- The reactants that initially present undergoes mutual reaction.
- The products, the aftermath of the reaction.
Here Carbon and oxygen are the reactants, the arrow symbol shows the direction of the reaction and the product here is carbon dioxide.