In the equation
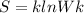 represents Boltzmann constant
represents Boltzmann constant
Answer: Option D
Step-by-step explanation:
Entropy is defined as the degree of randomness of a system. In the given equation S represents entropy of the gas, k represent Boltzmann constant and W represents number of micro states possible.
W or number of micro states means the number of ways atoms and molecules ca be arranged in a thermodynamic system. When number of micro states is more the degree of randomness namely the entropy is more.
In solid to liquid transformation the entropy increases. Similarly liquid to gas transformation also increases the entropy of the system increases.