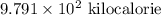Answer : The correct number of significant figures in scientific notation is
Explanation :
The conversion used from kilojoules (kJ) to kilocalories (kcal) is:
1 Joule = 4.184 Calorie
or,
1 kilojoule = 4.184 kilocalorie
As we are given that the energy 234 kJ. Now we have to convert it into kcal.
As,
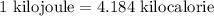
So,
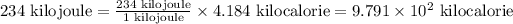
Therefore, the correct number of significant figures in scientific notation is