Answer:
d.) It decreases CO2 and H+ in the blood, increasing pH
Step-by-step explanation:
Carbon dioxide is eliminated from the body as a gas in exhaled air, during hyperventilation the volume of air breathed in and our increased above the normal, causing more
 to exit the body affecting the equilibrium between bicarbonate ion and carbon dioxide:
to exit the body affecting the equilibrium between bicarbonate ion and carbon dioxide:
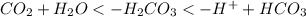
This change in the equilibrium (to the left) results in a pH rising because of the loss of H+.
I hope you find this information useful! Good luck!