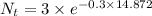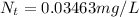Answer:
The concentration downstream reduces to 0.03463mg/L
Step-by-step explanation:
Initially let us calculate the time it is required to fill the lake, since for that period of time the pollutant shall remain in the lake before being flushed out.
Thus the detention period is calculated as
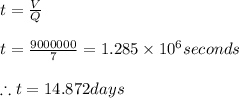
Now the concentration of the pollutant after 14.872 days is calculated as
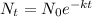
where
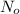 is the initial concentration
is the initial concentration
't' is the time elapsed after which the remaining concentration is calculated
k is the dissociation constant.
Applying values we get