Step-by-step explanation:
Ion-ion forces are defined as the forces that exist between oppositely charged ions.
Dipole-dipole interactions are defined as the forces which exist between positive end of polar molecule and negative end of another polar molecule.
Ion-dipole forces are defined as the forces that exist between a charged ion and a polar molecule.
London dispersion forces are defined as the forces that arise due to the development of temporary charges on the combining atoms of a molecule.
Hence, the given substances are classified as follows.
(a) HF - It is a covalent compound but due to the difference in electronegativity of hydrogen and fluorine there will be development of partial charges on both of them.
Hence, in a HF molecule there will be dipole-dipole forces.
(b)
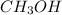 - There will also be development of partial charges due to the difference in electronegativity of oxygen and hydrogen atoms.
- There will also be development of partial charges due to the difference in electronegativity of oxygen and hydrogen atoms.
Hence, in a
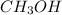 there will be dipole-dipole forces.
there will be dipole-dipole forces.
(c)
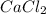 - It is an ionic compound. Hence, there will be partial positive charge on calcium and partial negative charge on chlorine atom.
- It is an ionic compound. Hence, there will be partial positive charge on calcium and partial negative charge on chlorine atom.
Hence, in a
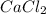 molecule there will exist ion-ion forces.
molecule there will exist ion-ion forces.
(d)
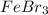 - It is an ionic compound. Hence, there will also exist ion-ion forces.
- It is an ionic compound. Hence, there will also exist ion-ion forces.