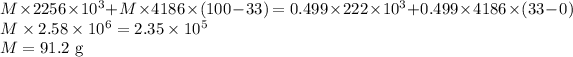Answer:
The mass of the steam is 91.2 g
Mass of the steam=91 grams
Step-by-step explanation:
Given:
- Mass of the ice=499 g
- Final temperature of the liquid water
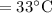
- Latent heat of fusion=
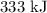
- Latent heat of vaporization =
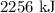
When steam is mixed with the ice then the heat loss by the steam will be gained by the ice so there will no overall heat gain or loss during the mixing
So According to question
Let M be the mass of the steam mixed with ice then we have