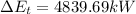Answer:
(1)
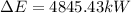
(2)
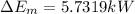
(3)
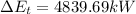
(4) q = 4839.69 kW[/tex]
Solution:
Using Saturated water-pressure table corresponding to pressure, P = 10 bar:
At saturated temperature, Specific enthalpy of water,
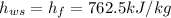
At inlet:
Saturated temperature of water,
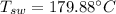
Specific volume of water,
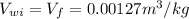
Using super heated water table corresponding to a temperature of
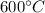 and at 7 bar:
and at 7 bar:
At outlet:
Specific volume of water,
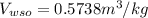
Specific enthalpy of water,
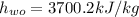
Now, at inlet, water's specific enthalpy is given by:
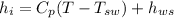
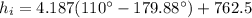
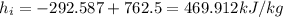
(1) Now, the change in combined thermal energy and work flow is given by:
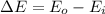
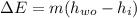
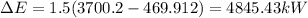
(2) The mechanical energy can be calculated as:
velocity at inlet,
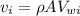
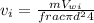
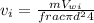
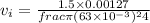
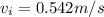
Similarly,, the velocity at the outlet,
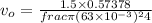
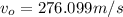
Now, change in mechanical energy:
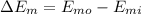
![\Delta E_(m) = m[((v_(o)^(2))/(2) + gz_(o)) - ((v_(i)^(2))/(2) + gz_(i))]](https://img.qammunity.org/2020/formulas/engineering/college/rvn8nyiskknvoxkpuyhl7dwa1uv8nsu2ee.png)
![\Delta E_(m) = 1.5[((276.099^(2))/(2) + 9.8(z_(o) - z_(i)) - ((0.542^(2))/(2)]](https://img.qammunity.org/2020/formulas/engineering/college/zp5m1sgxpa87awikqyzfxozs8garr8cmyc.png)
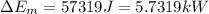
(3) The total energy of water is given by:
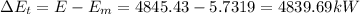
(4) The rate of heat transfer:
q =