Answer:
distance r from the uranium atom is 18.27 nm
Step-by-step explanation:
given data
uranium and iron atom distance R = 44.10 nm
uranium atom = singly ionized
iron atom = doubly ionized
to find out
distance r from the uranium atom
solution
we consider here that uranium electron at distance = r
and electron between uranium and iron so here
so we can say electron and iron distance = ( 44.10 - r ) nm
and we know single ionized uranium charge q2= 1.602 ×
 C
C
and charge on iron will be q3 = 2 × 1.602 ×
 C
C
so charge on electron is q1 = - 1.602 ×
 C
C
and we know F =
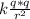
so now by equilibrium
Fu = Fi
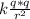 =
=
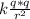
put here k =
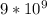 and find r
and find r
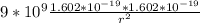 =
=
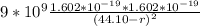
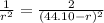
r = 18.27 nm
distance r from the uranium atom is 18.27 nm