Answer:
The kinetic energies are 0.46 eV and 0.78 eV.
Step-by-step explanation:
Given that,
Work function = 1.5 eV
Wavelength = 632.8 nm
We need to calculate the energy for red helium neon laser
Using formula of energy
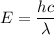
Put the value into the formula
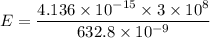
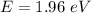
We need to calculate the kinetic energy
Using formula of K.E
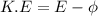
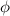 =work function
=work function
E = energy
Put the value into the formula
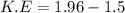
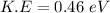
We need to calculate the energy for green helium neon laser
Using formula of energy
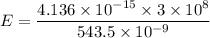
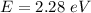
We need to calculate the kinetic energy
Using formula of K.E
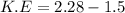
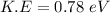
Hence, The kinetic energies are 0.46 eV and 0.78 eV.