93.75% of a material decays at the end of
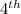 half-life.
half-life.
Step-by-step explanation:
The time needed by a material to reduce its initial amount into half is called half-life. It is generally used term in nuclear physics. This is to explain how fast unstable atom undergoes, or how long can stable atoms present, and radioactive decay.
Decay % of material after each half-life to be calculated as follows,
After one half-life- 50% original, and 50% product decayed.
After two half-life- 25% original, and 75% product decayed.
After three half-lives- 12.5% of original, and 87.5% of decay product.
After four half-lives- 6.25% original, and 93.75% of decay product.