Answer:
pH of acetic acid solution is 2.88
Step-by-step explanation:
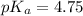
or,
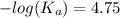
or,
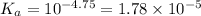
We have to construct an ICE table to determine concentration of
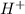 and corresponding pH. Initial concentration of acetic acid is 0.1 M.
and corresponding pH. Initial concentration of acetic acid is 0.1 M.
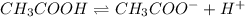
I(M): 0.1 0 0
C(M): -x +x +x
E(M): 0.1-x x x
So,
![([CH_(3)COO^(-)][H^(+)])/([CH_(3)COOH])=K_(a)](https://img.qammunity.org/2020/formulas/chemistry/college/74brdysvwy7r46jk4ep3s8pnqphb75330d.png)
or,
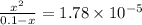
or,
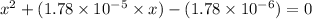
So,
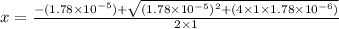 (M)
(M)
so,
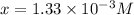
Hence
![pH=-log[H^(+)]=-log(1.33* 10^(-3))=2.88](https://img.qammunity.org/2020/formulas/chemistry/college/qo8txutj9cxpmtrhpru0216b8lu02ede8n.png)