Answer:
The sample of lithium occupies the largest volume.
Step-by-step explanation:
Given the densities for the four elements, we have the expression
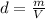 that shows the relationship between mass and Volume to express the density of an element.
that shows the relationship between mass and Volume to express the density of an element.
For each element we have:
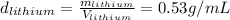
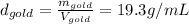
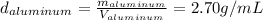
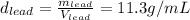
The problem says that all the samples have the same mass, so:
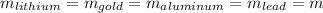
it means that m is a constant
Now, solving for the Volume in each element and with m as a constant, we have:
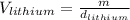
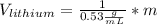
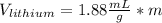
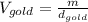
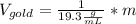
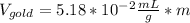
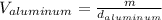
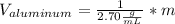
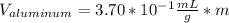
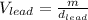
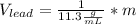
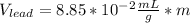
If we assume m = 1g, we find that:
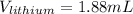
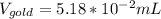
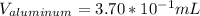
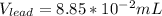
So we can see that the sample of lithium occupies the largest volume with 1.88mL
Note that m only can take positive values, so if you change the value of m, always will be the lithium which occupies the largest volume.