Answer:
The least amount of energy emitted in this case is 0.6 eV.
The corresponding quantum number n would be n=4.
The wavelenght asociated to the emitted photon would be 2.06
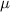 m, corresponding to the Infrared spectrum.
m, corresponding to the Infrared spectrum.
Step-by-step explanation:
For calculating the energy of an electron emitted/absorbed in an electronic transition of the hydrogen atom, the next equation from the Bohr model can be used:
![E=E_(0) Z^2 [(1)/(n_1^2)-(1)/(n_2^2)]](https://img.qammunity.org/2020/formulas/chemistry/college/a6nhrqc5vluf907af55udzgkuvb4net37y.png)
, where E is the photon energy,
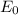 is the energy of the first energy level (-13.6 eV), Z is the atomic number,
is the energy of the first energy level (-13.6 eV), Z is the atomic number,
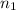 is the quantum number n of the starting level and
is the quantum number n of the starting level and
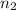 the quantum number n of the finishing level. In this case,
the quantum number n of the finishing level. In this case,
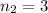 , and
, and
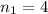 , because this excited level is the next in energy to n=3.
, because this excited level is the next in energy to n=3.
Considering that
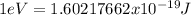 , and using the Planck equation
, and using the Planck equation
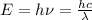 , you can calculate the wavelenght or the frequency associated to that photon. Values in the order of
, you can calculate the wavelenght or the frequency associated to that photon. Values in the order of
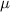 m in wavelenght belong to the Infrared spectrum, wich can not being seen by humans.
m in wavelenght belong to the Infrared spectrum, wich can not being seen by humans.