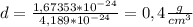Answer:
The density of the hydrogen atom is 0,4 g/cm3 and the density of the hydrogen nucleus is 7,8x10^14 g/cm3.
Step-by-step explanation:
Hydrogen is the first element of the periodic table
 .
.
The atomic number of an element tell us the number of protons (Z), and the atomic mass (A) tell us the number of protons plus neutrons. The number of electrons is always the same as protons. Therefore, for the hydrogen:
(Z): 1=# protons
(A): 1=#protons + #neutrons
Solving this system, we have for hydrogen:
#protons=1
#electrons=1
#neutrons=0
Density (d) is defined as the relation between mass and volumen:
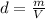 .
.
Density of the hydrogen nucleus:
In the nucleus we have the protons and neutrons, as the hydrogen doesn't have neutrons, we onlye have one proton in its nucleus. The mass of the proton is 1,67262 x
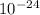 g, therefore the mass of the nucleus is 1,67262x
g, therefore the mass of the nucleus is 1,67262x
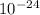 g.
g.
Will find the volume of the nucleus assuming it has a spherical shape. The radius of the hydrogen nucleous is 8 x
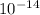 cm. To calculate the volume of an sphere we use the following equation:
cm. To calculate the volume of an sphere we use the following equation:
v =
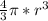
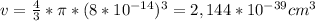
Finally, to calculate the density we only replace the mass and volumen in the density equation:
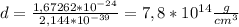
Density of the hydrogen atom:
In the atom we have all the electrons, neutrons and protons. For the hydrogen atom we have 1 proton and 1 electron. The mass of one electron is 9,1x
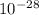 g. So, the mass of the hydrogen atom is 9,1x
g. So, the mass of the hydrogen atom is 9,1x
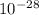 kg + 1,67262x
kg + 1,67262x
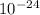 = 1,67353x
= 1,67353x
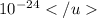 g.
g.
To find the volume we use the equation of the sphere, but now using the value of 1x
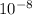 cm as radius.
cm as radius.
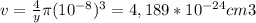
And the density will be: