Answer:
(D) 0.250 mole
Step-by-step explanation:
From molecular weight we know that 1 mole of P4O10 wights 284 grams. So
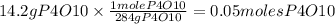
In order to form 1 mole of P4O10 it is necessary 5 moles of O2, so that the number of oxygen atoms are the same.
Knowing that 0.05 moles of P4O10 is formed we need 5 x 0.05 = 0.25 moles of O2