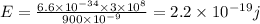Answer:
Energy of UV light
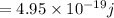
Energy of green light
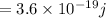
Energy of infrared light
Step-by-step explanation:
We have given the wavelength of UV light = 400 nm
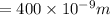 , wavelength of green light = 550 nm and wavelength of infrared = 900 nm
, wavelength of green light = 550 nm and wavelength of infrared = 900 nm
Speed of light
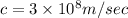
Plank's constant
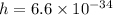
Energy of the signal is given by
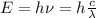
So energy of UV light
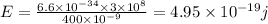
Energy of green light
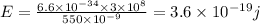
Energy of infrared light