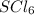Answer:
Step-by-step explanation:
So, the formula for the compound should be:
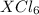
Now we assume that we have 1 mol of substance, so we can make calculations to know the molar mass of element X, as follows:
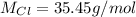
So we have that 6 moles weight 212.7g, and we can make a rule of three to know the weight of compound X:
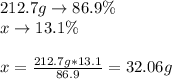
As we used 1 mol, we know that the molar mass is 32.06g/mol
So the element has a molar mass of 32.06 g/mol and an oxidation state of +6, with this information, we can assure that the element X is sulfur, so the compound is