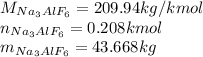Answer:
43.668 kg
Step-by-step explanation:
First we set the equation:
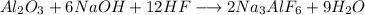
Now, we need to now the kmoles for each reactant:
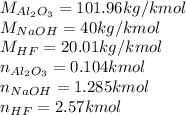
With this, we can see that the limit reactant is the aluminum oxide, so, with the equation for the reaction we know that 1 kmol of aluminum oxide, produces 2 kmol of cryolite, so we set a rule of three and see that 0.208 kmoles of cryolite are produced, the we proceed to calculate the mass: